Pursuing a Cure for Cancer
Currently, “chemo-radiation” treatments are well established in cancers of the head and neck, esophagus, lung, stomach, breast, brain, pancreas, rectum and uterine cervix. The ideal radiation sensitizer would reach the tumor in adequate concentrations and act selectively in the tumor compared to the surrounding normal tissue. It would have predictable pharmacokinetics for timing with radiation therapy and could be administered with every radiation treatment approach. The ideal radiation sensitizer would have minimal toxicity or manageable enhancement of radiation therapy.
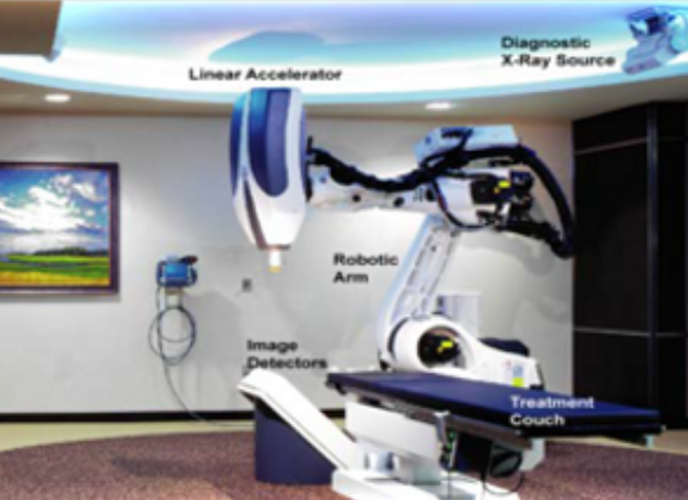

Shuttle’s Revolutionary Solutions
Our HDAC platform technology pipeline of products is designed to address the limitations of the current standard of cancer therapies. Because our proprietary technology activates the DNA damage response and expression of immune modulating proteins, they affect cancer cell killing by radiation and by the immune system. As a result of this, we are exploring our novel technology with immunotherapies. We have demonstrated proof-of-concept of our HDAC platform in preclinical models for a number of diseases and we are focused on developing this technology for solid tumor cancers.
In addition, we have included in our portfolio clinical-stage products that complement our overall vision to cure cancer using novel therapies in conjunction with radiation therapies. We utilized non-dilutive SBIR contracts to advance the radiation sensitizer Ropidoxuridine through a phase I clinical trial. we have signed a licensing agreement with the University of Virginia to develop and commercialize Heavy Oxygen IPdR, a proprietary approach for a proton specific radiation sensitizer, and have in-licensed the hypoxic radiation sensitizer Doranidazole for clinical testing in the US from Pola Pharma. We have discovered novel HDAC inhibitors in our laboratories.
